Abstract
Background: Ponatinib is a third-generation tyrosine kinase inhibitor (TKI) designed to potently inhibit native BCR::ABL1 as well as all single resistance mutations, including the T315I mutation. OPTIC (Optimizing Ponatinib Treatment in CP-CML, NCT02467270; ongoing) is a Phase 2 trial evaluating the efficacy and safety of ponatinib using a novel response-based dose-adjustment strategy in patients with CP-CML whose disease is resistant to ≥2 TKIs or who harbor T315I. The purpose of this strategy is to optimize efficacy and improve the safety of ponatinib in patients with highly resistant CP-CML. Results from OPTIC primary analysis demonstrated an improved risk:benefit ratio for ponatinib by using a response-based dosing strategy starting with 45 mg/d, followed by dose reduction to 15 mg/d upon attaining ≤1% BCR::ABL1IS. Here, we present a 3-year update of efficacy and safety outcomes from the OPTIC trial.
Methods: Patients with CP-CML resistant to ≥2 TKIs or with the BCR::ABL1 T315I mutation were randomized to ponatinib starting doses of 45 mg, 30 mg, and 15 mg once daily. Doses were reduced to 15 mg with attainment of ≤1% BCR::ABL1IS in the 45-mg and 30-mg cohorts. The primary endpoint was ≤1% BCR::ABL1IS at 12 months; secondary endpoints included molecular responses and safety outcomes, including arterial occlusive event (AOE) rates that were adjudicated prospectively by an independent review committee. Median time to response and probabilities of overall survival and progression-free survival by 36 months were estimated using the Kaplan-Meier method.
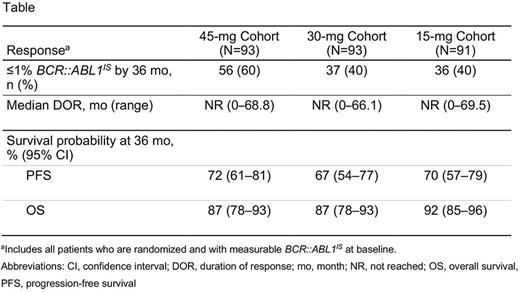
Results: A total of 283 patients were randomized (45 mg/30 mg/15 mg: n=94/95/94). The median age was 48 years (18‒81 years). Overall, 98% of patients had received ≥2 prior TKIs (55% ≥3); 99% stopped ≥1 prior therapy due to resistance; and 40% had ≥1 baseline mutations (23% T315I). Across cohorts, 61% of patients had a complete hematologic response or worse as best response to last prior TKI. As of May 2022, 60%, 40%, and 40% of patients in the 45-mg, 30-mg, and 15-mg cohorts, respectively, reached ≤1% BCR::ABL1IS by 36 months (Table). Of the patients with T315I mutations, 64% (16/25), 25% (5/20), and 16% (3/19) attained ≤1% BCR::ABL1IS by 36 months versus 59% (39/66), 44% (32/73), and 46% (33/71) of patients without T315I in the 45-mg, 30-mg, and 15-mg cohorts, respectively. Among patients who reached ≤1% BCR::ABL1IS at any time, the proportions of patients who lost response at any time in the 45-mg and 30-mg cohorts were 27% (15/56) and 24% (9/38), respectively; T315I was present in 60% (9/15) and 33% (3/9) of patients who lost response, respectively. In the 45-mg and 30-mg cohorts, 27% (12/45) and 11% (3/27) patients who had per protocol dose reductions to 15 mg upon attaining ≤1% BCR::ABL1IS had their dose re-escalated after loss of ≤1% BCR::ABL1IS response; 75% (9/12) and 67% (2/3) of these patients regained ≤1% BCR::ABL1IS, respectively. The median time to regain response after dose re-escalation among patients who attained ≤1% BCR::ABL1IS response was 126 days (95% CI, 39-167) in the 45-mg cohort; median time to regain response in the 30-mg cohort was not applicable because there were only 2 responders. Among patients with T315I, 89% (8/9) in the 45-mg cohort and 67% (2/3) in the 30-mg cohort had dose re-escalations after loss of ≤1% BCR::ABL1IS response; 75% (6/8) and 50% (1/2) of these patients, respectively, regained a ≤1% BCR::ABL1IS response. Most common grade ≥3 treatment-emergent adverse events were thrombocytopenia (27%), neutropenia (18%), hypertension (9%), and anemia (8%). Grade ≥3 AOEs were reported in the 45-mg cohort (6%), 30-mg cohort (6%), and 15-mg cohort (4%) and were similar to reported rates in the primary analysis of OPTIC (45 mg, 5%; 30 mg, 5%; 15 mg, 3%).
Conclusions: Results from the 3-year follow-up of the Phase 2 OPTIC study provide the robust long-term efficacy and manageable safety profile of ponatinib in a highly resistant CP-CML population. Consistent with results from the primary analysis, a ponatinib starting dose of 45 mg/d with reduction to 15 mg/d upon attainment of ≤1% BCR::ABL1IS provided the optimal benefit:risk ratio.
Disclosures
Cortes:Takeda: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Gilead: Consultancy; Sun Pharma: Consultancy, Research Funding; Biopath Holdings Inc: Consultancy, Current equity holder in private company; Abbvie: Consultancy, Research Funding; Forma Therapeutic: Consultancy; Novartis: Consultancy, Honoraria, Research Funding. Deininger:Galena Biopharma: Consultancy, Honoraria; Pfizer Inc: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Blueprint Medicines Corporation: Consultancy, Honoraria, Research Funding. Lomaia:Bristol Myers Squibb: Other: Travel, Accommodation, Expenses ; Fusion Pharma: Speakers Bureau; Pfizer: Other: Travel, Accommodation, Expenses , Speakers Bureau; Novartis: Other: Travel, Accommodation, Expenses , Speakers Bureau. Moiraghi:Takeda: Speakers Bureau; Pfizer: Speakers Bureau; Novartis: Speakers Bureau. Sutton:Roche: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Membership on an entity's Board of Directors or advisory committees. Pavlovsky:Novartis, Pfizer, BMS, Pint Pharma: Speakers Bureau; Novartis, Pfizer: Membership on an entity's Board of Directors or advisory committees. Chuah:Korea Otsuka Pharmaceutical: Honoraria; Otsuka [Philippines] Pharmaceutical: Honoraria; Steward Cross: Korea Otsuka International Asia Arab: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Pfizer: Other: Travel, Research Funding; Novartis: Honoraria. Sacha:Adamed: Consultancy, Honoraria; Incyte: Consultancy, Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Speakers Bureau; BMs-Celgene: Honoraria, Speakers Bureau; AOP Orphan: Honoraria, Speakers Bureau; Angelini: Honoraria, Membership on an entity's Board of Directors or advisory committees. Lipton:Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; ARIAD Pharmaceuticals: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding. McCloskey:AbbVie, CTI BioPharma, and Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie, Amgen, Bristol Myers Squibb, Incyte, Jazz Pharmaceuticals, Stemline, and Takeda: Speakers Bureau. Hochhaus:Bristol Myers Squibb: Research Funding; Pfizer: Research Funding; Novartis: Research Funding; Incyte: Research Funding. Rousselot:Novartis: Consultancy; Bristol Myers Squibb: Consultancy; Pfizer: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; Takeda: Consultancy. Rosti:BMD: Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees, Other: Steering Committee , Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Other: Steering Committee , Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Steering Committee , Speakers Bureau. de Lavallade:Pfizer: Honoraria; Incyte: Honoraria, Research Funding; Novartis: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding. Rojas:Novartis: Other: Personal fees; Roche: Other: Personal fees; Janssen: Other: Personal fees; AstraZeneca: Other: Personal fees. Turkina:Fusion Pharma: Speakers Bureau; Pfizer: Other: Travel, Accommodation, Expenses , Speakers Bureau; Novartis: Other: Travel, Accommodation, Expenses , Speakers Bureau. Talpaz:IMAGO: Consultancy; Novartis: Consultancy, Other: Grant/research support ; BMS: Consultancy; Kirin: Membership on an entity's Board of Directors or advisory committees; Takeda: Other: Grant/research support ; SDp: Membership on an entity's Board of Directors or advisory committees. Mauro:AbbVie, Bristol Myers Squibb, Novartis, Pfizer, Takeda: Consultancy, Honoraria, Other: Travel, accommodation, expenses , Research Funding; Sun Pharma/SPARC: Research Funding. Lu:Takeda: Current Employment. Vorog:Takeda: Current Employment. Apperley:Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Honoraria, Research Funding, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal